Since the threads inside of a hot-dip galvanized nut are bare metal, will they corrode?
The short answer to this question is no. However, there are many variables with regard to the protective coating that prevents the internal threads of a galvanized nut from corroding. In this FAQ, we will explore some of the variables brought about by this question. One of the first issues to address is the question of why the internal threads of a galvanized nut are bare metal to begin with. Hot-dip galvanizing is a process in which bolts and fasteners are coated with zinc to prevent them from corroding. Because the threads of a galvanized nut must accommodate the added thickness of the galvanized bolt, the nuts must be tapped oversized. The threads are tapped oversized after the nut has been galvanized, which leaves the internal threads as bare metal. This process is stated in the ASTM A563 specification which covers nuts: “4.7.5 – Hot-dip zinc-coated nuts shall be tapped after zinc coating.”
Now that we know why the threads inside of a nut are bare metal, we can explore the reasons as to why they do not corrode when used with a galvanized bolt. The simplest explanation as to why the internal bare threads of nuts will not corrode is due to the zinc coating’s cathodic properties. The zinc on the mating galvanized bolt’s threads provides a sacrificial coating that prevents the bare threads of the nut from corrosion.
Cathodic Protection
Steel begins to corrode when it is immersed in an electrolyte or exposed to surface moisture causing differences in electrical potential to the exposed steel. Because of this, negatively charged electrons begin to flow from anode to cathode and the iron atoms in the anode area become positively charged ions. The table below shows a number of metals that are arranged in order of electrochemical activity in the presence of an electrolyte (in this case the electrolyte is sea water). The metals are that higher up on the scale are able to provide cathodic protection for the metals below them. As is shown below, zinc is higher on the scale compared to aluminum, thus it will provide protection for the steel.
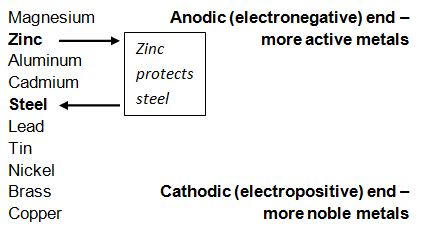
Chart from Galvanizers Association of Australia.
The chart above shows that Magnesium, Aluminum and Cadmium should also protect steel, however, due to type of reactivity, lack of effectiveness and economic/environmental issues, zinc is the best form of protection in regard to steel. As long as the galvanized coating remains on the bolt, the cathodic protection will continue to protect the bare threads on the nut.




I have a question regarding the durability/effectiveness of alternative coatings to hot dip galvanizing. We have bare steel rods that will have threads cut to be used as foundation anchor bolts. Due to schedule constraints, hot dip galvanizing is not an available option. The current thought is to use zinc rich paint to coat the newly cut threads to protect against corrosion. Do you know if this is a common practice and if there are any concerns with this approach? Are there any other alternatives we should consider as well? Thank you for your assistance!
@Allan- We can’t speak to how commonly this is done, but since zinc-rich paint is commonly available, we’d guess it is done more that we are aware. Our only concern is that the zinc paint will not protect nearly as well as the hot dip zinc process. It will be better than bare steel, but exactly how much protection it offers by itself, we are not sure. There are many coating alternatives, but all take time, so if you do not have time for HDG, you likely would be limited to coatings that can be applied in the field, and those we are not familiar with.
Is there any other solution to protect the threaded portion of anchor bolts from corrosion rather than using bright zinc spray.
@Nishad- Before the bolts are installed, there are many coating options, the most common being hot dip galvanizing. Once the bolts are installed, your options are limited to portable delivery devices like spray cans, brushes, etc. We are less familiar with all those options, but zinc spray and paint are of course among the most common.
We use threaded rods grade 8.8 ( equivalent 92.8 Ksi). How can we provide corrosion protection without build up on threads that will effect tightening of the nuts ?
@David- The two ways to have corrosion protection are to use a corrosive resistant steel to start with (like stainless or Core-ten), or to coat the steel with something (like zinc or some other compound). Because you already have the steel, and it is not inherently corrosion resistant, the only other alternative is to coat it. All coatings will effect the nut tightening somehow, some more than others. Without knowing more about the specifics of your application, I can’t really make any sort of recommendations, other than to say there are several coating options out there, and there are pros and cons to each. A coatings engineer might be able to provide better insight.